Axial - Observations #26
Life sciences reflections
More well thought out work can be found at — https://axial.substack.com/
Axial partners with great founders and inventors. We invest in early-stage life sciences companies often when they are no more than an idea. We are fanatical about helping the rare inventor who is compelled to build their own enduring business. If you or someone you know has a great idea or company in life sciences, Axial would be excited to get to know you and possibly invest in your vision and company . We are excited to be in business with you - email us at info@axialvc.com
Observations #26
A set of ideas and observations from a week’s worth of work analyzing businesses and technologies.
Scale in biology
During grad school, one of my professors, David Drubin, spent a whole session of class to discuss numerical numbers of size, energetics, force, and other features in biology. That class has always stuck with me. Biologists tend to think in terms of genetic or biochemical rules or through memorization of a pathway. But more often than not, we forget the simple things like the relative size of mRNA or diffusion coefficients. However, these numbers can be incredibly valuable to come to a unique insight that could ultimately lead to a new discovery, tool, or medicine.
A great resource for these types of numbers is Cell Biology by the Numbers:
How big are viruses?

How big are the components of the central dogma?

The speed of transcription versus translation?

The relative size of mRNA versus the protein it encodes for?

How long does the cell cycle take?

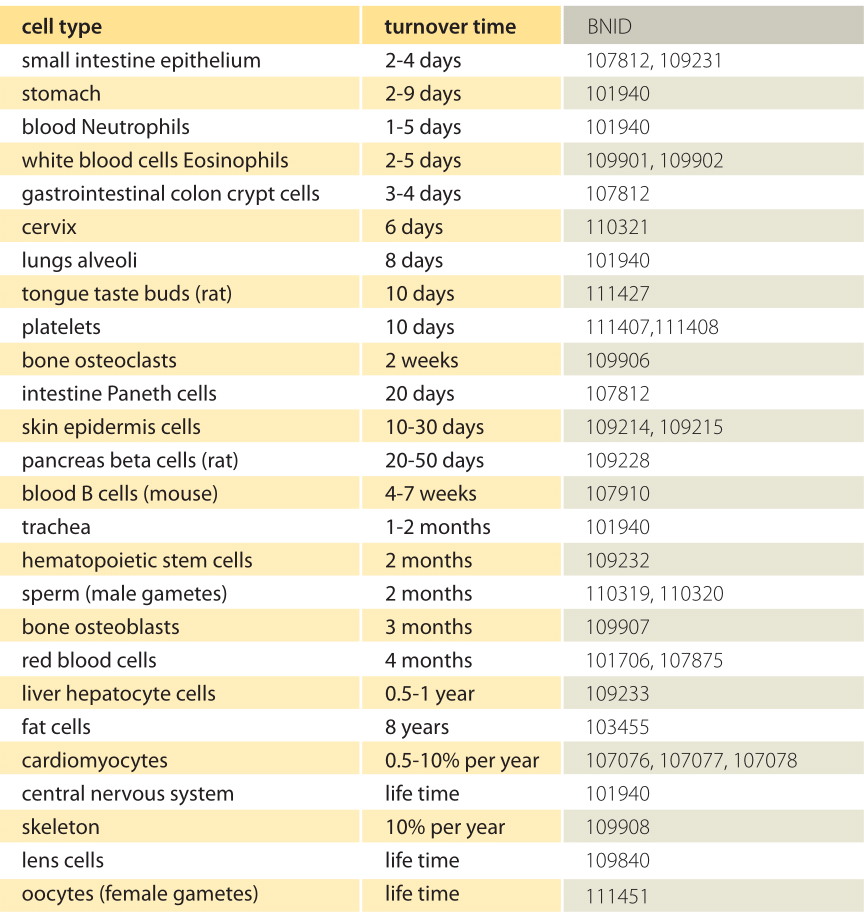
What are the turnover times for human cells?
What is the half-life of mRNA?

What are the diffusion coefficients in the cell?

The industrial R&D lab
A recent article on industrial labs did a good job pinpointing their downfall around the 1970s to the slow fall of AT&T Bell and Xerox’s monopolies. Bob Metcalfe (invented Ethernet, 3Com, Metcalfe’s Law) gave in an interview in 2006 touching on the same point:
“One of the few things government should do is finance research, because - I have learned from many years - the only companies that can afford to do research are monopolies. Real companies cannot afford to do research other than monopolies. And there are some famous ones. The telephone monopoly: Bell Labs. The computer monopoly: Watson Labs. The copier monopoly: Xerox Parc. And on it goes. In retrospect the monopolies aren't worth it for the research they do. It's nauseating how much we hear about how cool Bell Labs was, but other than the transistor and Unix and the princess telephone, what did we get for all that money? And then for years AT&T as a monopoly sat on innovation and IBM after that, and Xerox after that - it's just not worth it, so let's kill those monopolies. And if we need research, have it done at research universities. And the other spin I would offer there, as a practitioner of technological innovation, I worry about technology transfer - how do you get technology transferred from the lab into the marketplace. And the best way to do that is with people; and it is the business of universities to graduate people. So let's do our research there, and I think the ARPANet is a great example where the government financed the research.”
The article lays out the history of industrial labs and comes to a similar conclusion as Metcalfe, but how do we get technology transferred from the lab to a company? A lot of the focus is on university incentives or even reviving industrial labs somehow. The former might be equivalent to the Catholic church in the 1500s and the latter might be very inefficient at commercializing work since monopolies have their own set of incentives to not be disrupted. Metcalfe alludes to this, but the answer to the downfall of industrial labs and maybe an overall decrease in progress, is to empower the individual. This is the mission of Axial: to help great inventors form their own companies.
Basket trials in oncology
Basket trials are a type of a clinical trial to test how a drug works in different disease subtypes concurrently. The idea was pioneered at MSKCC. They allow patients with different disease subtypes to enroll in the same trial and receive the same investigational drug. These trials are very useful to gain approval across many indications with one trial - this helps patients gain access to new medicines and helps companies more quickly get to multiple markets. Basket trials are particularly useful for genomically-defined diseases. Cancer is probably where genomics has had the most impact on so far. As a result, most if not all basket trials have been in oncology. With the wider adoption of genomics, tumor-agnostic drug development is growing in prominence to help create more targeted cancer therapies and offers signals for what is in store for autoimmunity.
What are the features of a basket trial?:
Early-stage, single-arm studies
Relies on large signals of activity from a molecular feature instead of anatomical-based tumor type
Around 20-30 patients per sub-study (a positive signal in one sub-study or basket can often lead to confirmation of the signal across the larger group)
Ideally uses Simon’s two-stage design with futility stopping


The initial proof-of-concept for developing medicines in a tumor-agnostic way was the development of imatinib for treatment of chronic myelogenous leukemias (CML) with the BCR/ABL translocation. Then drugs targeting HER2 for breast cancer, BRAF V600 for melanoma, and EGFR for lung cancer were developed. Overtime as genomics characterized tumors at a higher resolution, we moved from measuring single mutations to profiling a larger set of genes (i.e. Foundation Medicine). This has then enabled the use of basket trials in oncology because tumors can be characterized by more than their location in the body. This has led to a mini-golden age for rare cancers because patients can be pulled together based on the underlying mutations of their cancer. In the past, patient recruitment for rare cancers was just as difficult as any rare disease. The progress of basket trials have been powered by important studies that have produced medicines like pembrolizumab (Keytruda) for mismatch-repair-deficient advanced cancers, vemurafenib (Zelboraf) for the treatment of melanoma with the BRAF V600E genetic mutation, and larotrectinib (Vitrakvi) for solid tumors with NTRK fusions:
BATTLE trial (2011) (Biomarker-integrated Approaches of Targeted Therapy for Lung Cancer Elimination) - a prospective, biopsy-mandated, biomarker-based, adaptive randomized clinical trial platform for patients with advanced NSCLC. This trial served as a cases study on adaptive clinical trial design for future trials where enrollment is adapted from the full population to focus on patients who seem to be benefiting.
VE-BASKET trial (2015) - testing the efficacy of vemurafenib (inhibitor of BRAF V600 kinase) in non-melanoma cancers. This was the first published basket study where patients across multiple cancer types were enrolled based on their genetic profile.
Keytruda trial (2017) - testing Keytruda (PD-1 inhibitor) in patients with mismatch-repair-deficient tumors confirmed tumor-agnostic activity. This study led to the approval of the drug for any mismatch-repair-deficient advanced cancer patient. This was the first time a drug was approved based only on genomic characteristics of a patient’s tumor.
Pediatric NCI-Match trial (2017) - first basket trial solely focused on pediatric cancers
Larotrectinib trial (2018) - study of larotrectinib efficacy for TRK-fusion positive cancer. This study showed the power of using both adult and pediatric cancers in a basket trial to speed up approval.
The next generation of basket trials will require improvements in biomarker selection, patient matching, and other tools. However, these trials are incredibly valuable to enroll patients with rare diseases that have common molecular features:
Basket trials needed better statistical design. Single arm sub-studies need a response rate endpoint that can be challenging to define given the lack of historical controls for the diverse patients and diseases in each basket.
New biomarkers and improved selection, which will lead to better patient matching and enrollment. Particularly in cancer, there is still a high degree of heterogeneity across tumors in each basket.
Can progress in new and maybe rare genomic biomarkers be expanded to larger cancer types? The Keytruda trial is an early signal that this can be done.
Moving beyond small molecule inhibitors for specific mutations. The Keytruda trial again is an early signal of the power of bringing the full arsenal of medicine from antibodies and beyond to basket trials.
Bringing the power of basket trials to autoimmunity. Who is building the Blueprint Medicines for autoimmunity? They would probably have to target autoreactive B-cells.
Ultimately, basket trials are driving a revolution in oncology to bring more precision to patients and increase the efficiency of testing new drugs.

Reimbursing for AI
About a day ago, the CMS approved paying for Viz.ai’s AI software to detect strokes (Viz ContaCT) and coordinate care for the patient - https://www.prnewswire.com/news-releases/vizai-granted-medicare-new-technology-add-on-payment-301123603.html This is incredible news for patients as strokes are the largest cause for long-term disabilities. Moreover, this approval acts as a case study and precedent on how AI products will be priced and used in the US healthcare system; the full analysis/reasoning to pay for Viz.ai is here and starts at page 528 - https://s3.amazonaws.com/public-inspection.federalregister.gov/2020-19637.pdf
In short, Medicare will pay up to $1,040 per use of the software in patients with suspected strokes. The premise is that intervention time for a stroke patient greatly decreases brain damage and disability potential. Moreover, Viz’s algorithm could reduce overall times for coordinate care. This news is just the beginning with radiology, triaging, and other fields next.

